AI-Med Collaboration Platform
Project description
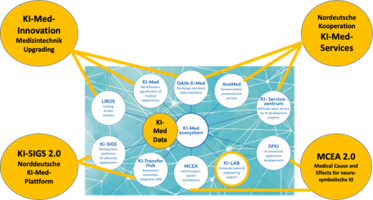
The BMFTR-funded project "KI-Med Collaboration Platform" (KiMeKo) aims to enhance the development of AI-based medical products by bundling competencies of numerous northern Germany research and development initiatives in form of standardized services and tools into a sustainable AI-med ecosystem. This ecosystem is dedicated to boost development and implementation of regulatory-approved AI-med products and driving the competitiveness of the northern Germany healthcare sector on an international scale by providing tools, methods, and services for development of AI-med products via the platform of the KI-Med-Ökosystem Nord.
Short Facts
- Homepage: https://kimeko.digital-hub.sh/
- Project period: 07/01/2024 – 12/31/2027
- Funder: Bundesministerium für Forschung, Technologie und Raumfahrt (BMFTR) [Registration no. 01|S24056A]
- Total funding: 3.03 m. €
Collaboration Partners
Subproject 3: AI-Med fusion of measurement and monitoring data
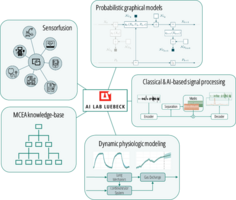
Medical devices generate a multitude of vital data used for diagnostics, therapy, quality monitoring, and fault tolerance. By automatically fusing and analyzing this data from various devices, complex predictive models by means of digital twins can be developed to support clinicians and users by, e.g., providing patient specific insights and improving personalized therapy. Joining forces with the Institute of Telematics (ITM, UzL) and the Universität Bremen (UB), as well as the German Research Center for Artificial Intelligence (DFKI) Bremen and the Fraunhofer-Einrichtung für Individualisierte und Zellbasierte Medizintechnik (IMTE), we work on building sustainable, generalizable, and reusable tools for sensor fusion that will be integrated to the platform.
By relying on probabilistic, graphical models, e.g., factor graphs and by integrating these models with Medical Cause and Effect Analysis (MCEA), we provide tools to build robust medical digital twins, that enable transparent reasoning, e.g., on the patient individual health status, keep track of modeling and measurement uncertainty, and incorporate clinical guidelines and knowledge to meet the high-stake requirements of the medical domain.
To enable the automatic sensor fusion required for the development of those digital twins, SDC-based tools according to the IEEE 11073 standard will be provided and extended to work with our probabilistic framework in order to allow the diverse medical devices of the clinical landscape to connect.
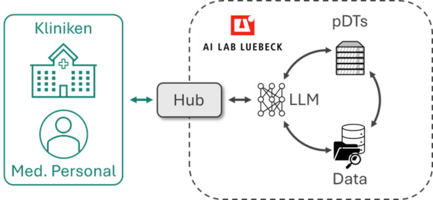
The proposed methods and tools will be validated by the implementation of partial digital twins for two use-cases, i.e., mechanically ventilated intensive-care patients and intelligent operating rooms at the LIROS (Fraunhofer IMTE). These will additionally be integrated to the platform by hosting them as services to allow stakeholders to interact and request specific simulations and results through LLMs and the model context protocol (MCP).
Members
Philipp Rostalski

Gebäude 19
philipp.rostalski(at)uni-luebeck.de
+49 451 3101 6200
Dimitrios Karachalios

Gebäude 19
dimitrios.karachalios(at)uni-luebeck.de
+49 451 3101 6235
Marlin Siebert

Gebäude 19
m.siebert(at)uni-luebeck.de
+49 451 3101 6220